Identify the Lewis Acid and Lewis Base
1 Circle the Lewis Acids and put box around the Lewis bases_ Na NH BF3 Ptz- AICl. 20 F2 F3 F4 F5 F6.
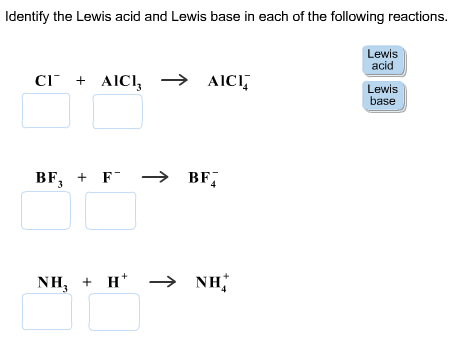
Identify The Lewis Acid And Lewis Base In Each Of The Following Reactions Home Work Help Learn Cbse Forum
A Fe3 is a Lewis acid.

. Therefore it is going to be electron deficient while a Lewis base is going to be able to donate a pair of electrons. AlBr3 is the Lewis base and NH3 is the Lewis acid. Both acids and bases are important aspects when we study chemistry.
Lewis acids are those species that contain an empty orbital which make it able to accept a pair of electrons from a Lewis base to form a covalent bond that is called Lewis adduct. This is big hint. Cl- BCl3 BCl4- Lewis acid.
Lewis bases are electron donors. Lewis acids - accept an electron pair. C Hg2 is a Lewis acid.
Know the definition. F e 3 2 H 2 O F e O H 2 H 3 O a F e 3 is the Lewis acid. Need help with chemistry.
I suppose H could be the Lewis acid here. To identify the lewis acid in the Lewis base among the reactant in the following reactions we need to recall the definitions of lewis acids and lewis bases. 4CO Ni NiCO.
Identify the Lewis acids and Lewis bases in the following reactions. Water is a Lewis base here. In a complex ion we have a central atom often consisting of a transition metal cation which acts as a Lewis acid and several neutral molecules or ions surrounding.
Lewis base v OHT C. Ag is the Lewis acid and NH3 is the Lewis base. Identify the Lewis acid and Lewis base from among the reactants in each of the following equations.
Lewis is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adductA Lewis base then is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to. Lewis base - donate an electron pair. I dont know why that is not the correct answer.
Questions to be asked. Also period 3 and up are able to expand their octet allowing them to accept more electrons because they a empty d orbital. In order to identify lewis acid and lewis base we have two consider.
A Lewis acid named for the American physical chemist Gilbert N. Part A Fe CIO43 s 6 H20 1 Fe H203 aq 3 C102 aq O Fe C1043 Lewis acid is O 6 H2O Submit Request Answer Part B O Fe C1043 Lewis base is 6 H20 1 Submit Request Answer Part C CN- aq H20 1 HCN aq OH aq O CN Lewis acid is. The acid-base theory of Brønsted has been used thoroughly in the history of acid and base.
Lewis acid are electron acceptors. A Lewis acid is a species that can accept an electron pair whereas a Lewis base has an electron pair available for donation to a Lewis acid. K 6H2O K H2O6 Lewis acid.
Fe HO Agt HzS CHCOO- 2 Identify the Lewis acid and Lewis base in the following equation. Ag is the Lewis base and NH3 is the Lewis acid. C H is a Lewis acid since it can accept a pair of electrons.
Lewis Bases will have a lone pair of electrons in their valence shell. B Cyanide is a Lewis base. Consider the reaction between NaOH and HCL Assign each reactant as an acid Or base under the Arrhenius Bronsted-Lowry and Lewis definitions and justify your assignment Explain.
A OH - is a Lewis base since it can donate its lone pair of electrons. H OH- H2O Lewis acid. Complex ions are examples of Lewis acid-base adducts.
Both HgI2 and HgI4 2- are Lewis acids. Neither - - Pl3 D. লইস এসড কষর চনর উপয super trick to identify lewis acid and base লইস এসড ও লইস কষরক AlCl3.
1 Identify the Lewis acid and Lewis base in the following equation. Lewis bases are those species that have a lone pair of electrons to donate to lewis acid to form a covalent bond that is called Lewis adduct. Identify each substance as Lewis acid Lewis base both or neither.
Part B AlBr3NH3H3NAlBr3 a. Part A Agaq2NH3aqAgNH32aq a. A lewis acid is going to be able to accept a pair of electrons.
B F - i s a Lewis base since it can donate a pair of electrons. Identify the Lewis acid and Lewis base among the reactants in each of the following reactions. Lewis acidbase motif is one of the most applicable theories and it extends the denotation of acids and bases beyond H and OH- ions.
Identify the Lewis acid and Lewis base from among the reactants in each of the following equations. D BCl 3 is a Lewis acid since it can accept a pair of electrons. Lewis acid - v Cu A Moving to another question will save this response.
Now what you need to look for in the given compound is whether the compound is capable of donating electron pairs or accepting them for example.
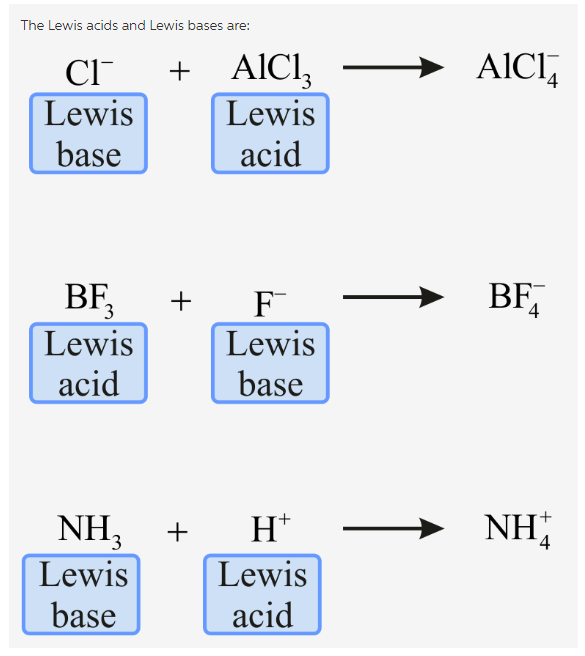
Identify The Lewis Acid And Lewis Base In Each Of The Following Reactions Home Work Help Learn Cbse Forum

Lewis Acids And Bases Chemistry Steps

Lewis Acids And Bases Chemistry Classroom Chemistry Education Chemistry Lessons
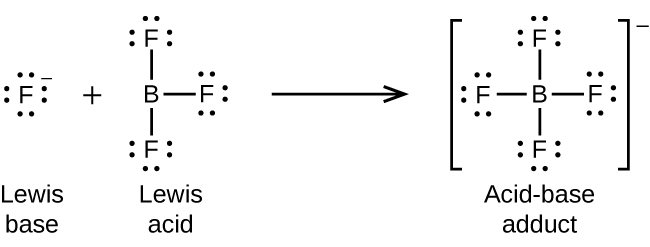
15 2 Lewis Acids And Bases Chemistry

Lewis Acids And Bases Chemistry For Non Majors

Lewis Acid And Base Definitions With Examples
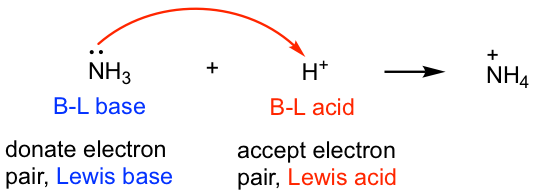
3 5 Lewis Acids And Lewis Bases Chemistry Libretexts
Illustrated Glossary Of Organic Chemistry Lewis Acid
Illustrated Glossary Of Organic Chemistry Lewis Acid
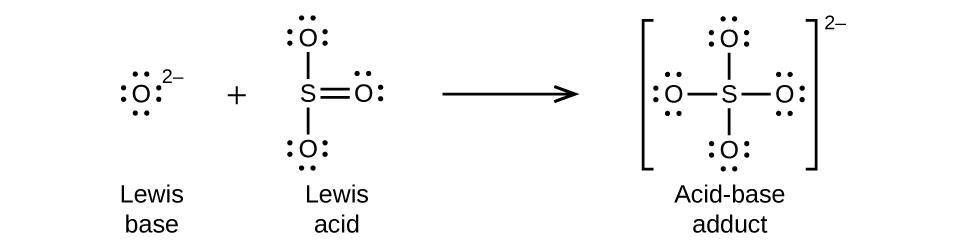
15 2 Lewis Acids And Bases Chemistry
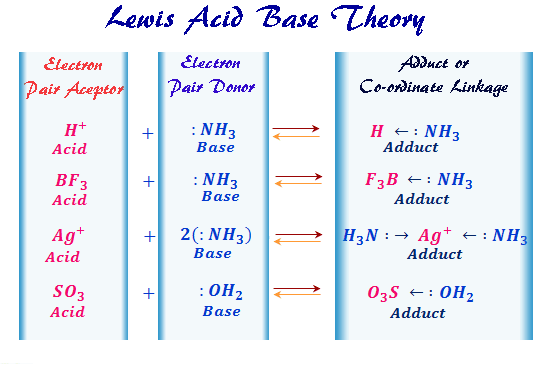
Lewis Acids Bases Definition Theory Properties Examples

Lewis Acids And Bases Definition Properties Examples Reactions Uses Applications Of Lewis Acids And Bases
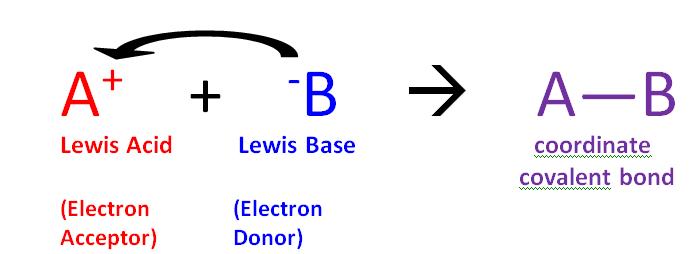
1 12 Lewis Acids And Bases Chemistry Libretexts

18 1 Lewis Theory Of Acids And Bases Hl Youtube
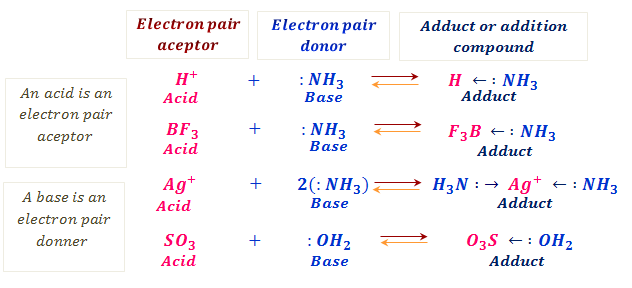
Example Of Lewis Acid Base Each Of The Concepts Had Its Own By Chemistry Topics Inorganic Chemistry Topics Medium

Lewis Acids And Bases Chemistry Steps



Comments
Post a Comment